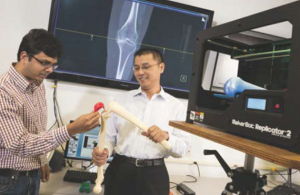
Image to implant solution for personalised medical devices
PLM (product lifecycle management) software enables a regulatory-compliant, cost-effective and streamlined process chain for planning, design and manufacture of patient-matched medical devices.
The demand for more “personalized” orthopedic treatments and therapies is growing rapidly. With the help of today’s integrated design, manufacturing and data management software technology, medical device manufacturers are now able to design and produce personalized orthopedic therapies optimized for an individual patient, making “personal” orthopedic medicine more widely accessible.
Patient-matched implants and surgical instruments result in better alignment of implants during surgery and reduced Incidence of subsequent corrective surgeries. With the help of an “Image-to-Implant” solution, enabled by Siemens PLM Software, medical device companies can develop personalized products and therapies that automate what is known in traditional manufacturing as an “engineer-to-order” process.
Until recently, most medical devices (such as surgical instruments or implants) have been designed for mass manufacturing and produced in a limited range of standard sizes and shapes that must be surgically “fit” to the patient. While this traditional approach is well attuned to support standard-offthe- shelf manufacture, it is not flexible enough to support the design and manufacture of personalized medical devices in a “mass customization” mode.
The Siemens Image-to-Implant solution is based on an MRI or CT image of a patient’s anatomy, so that the orthopedic implants and instruments are made to fit the patient rather than the reverse. By designing implants and surgical instrumentation (including the various cutting guides, fixtures and attachments needed to perform the surgery) that conform to a patient’s anatomy, manufacturers can produce optimized devices that reduce the need for more highly invasive surgeries, preserve more tissue, simplify surgical procedures, shorten patient recovery times and reduce the occurrence of revision surgeries.
Medical device manufacturers, doctors and patients can all benefit from personalized orthopedic therapies. But to bring these higher-value solutions to market, medical device manufacturers must move beyond conventional engineering practices to a more automated and collaborative engineer-to-order process.
With the help of advanced planning, a surgeon can implant a “template” that precisely fits the patient, according to an Emerging Technology Report on “Total Knee Replacement Using Patient-Specific Templates.”* The report lists the benefits of this technology, including:
- Improved alignment
- Decreased operative time
- Increased patient throughput
- Decreased instrumentation
- Reduced risk of fat embolism and intraoperative bleeding due to minimal bone removal (i.e., no intramedullary canal reaming)
- Decreased tissue loss
- Shorter recovery
- Reduced postoperative pain
- Decreased incidence of infection
- Lowered costs
3D printing as an emerging production alternative
3D printing, or additive manufacturing as it is referred to in certain circles, is increasingly becoming a mature option for product manufacturing in addition to rapid prototyping. 3D printing relieves some of the complexities with manufacturing complicated surface shapes and enables automation of CAM and CMM inspection processes. With 3D printing, you can also create shapes that are impossible to achieve with subtractive (CNC) or formative (casting, molding) methods.
For example, you can create internal structures such as lattices that enable the creation of lightweight yet strong parts and surfaces with intricate detail textures that improve part function and ergonomics. Siemens pioneered the use of 3D printing for production of personalized in-the-ear hearing aids.
3D printing is ideally suited for the personalized medical device industry for several reasons. Organic shapes are typically more complicated than engineered parts. The personalization of medical devices results in customized shapes but does not increase the complexity of manufacturing. Patient data derived from CT or MRI images can be used to directly create personalized devices using discrete polygonal mesh surface representations that allow the efficient processing of complicated organic shapes while avoiding the complex problem of converting to NURBS surfaces. The mesh representation can be directly used to derive tool paths for CAM in a simplified and automated manner. This also enables a high degree of automation within PLM systems, helping to integrate the entire engineer-to-order process chain.
Designed surface details can enable better osseointegration of implants. Lightweight latticed implants can reduce additional load on patients. With improvements in equipment, materials and processes, 3D printing has the potential to revolutionize the medical device industry.
Regulatory compliance for medical devices
In addition to helping medical device manufacturers personalize their products, PLM systems are also essential tools for tracking, monitoring and complying with the abundance of regulations imposed on these companies.
Unless medical device companies approach compliance from a holistic perspective, they will continue to spend too much valuable time and resources trying to mitigate the effects of noncompliance. As a result, regulatory compliance must be an integral part of the medical device lifecycle.
Using PLM can help you reduce and even eliminate many of the common causes for FDA nonconformance indications to medical device companies during the regulatory approval process for new product introductions.
Teamcenter® enables companies to maintain and manage their product and process knowledge in a globally accessible system. The system’s ability to capture audit trails, maintain electronic signatures and facilitate instant information retrieval is closely attuned to the medical device industry’s compliance management needs.